One drug, 166 patents and $208-billion sales
Down To Earth
|March 01, 2023
Arthritis drug Humira is a stark example of how the US patent regime allows drug firms to drain patients and the health system
-
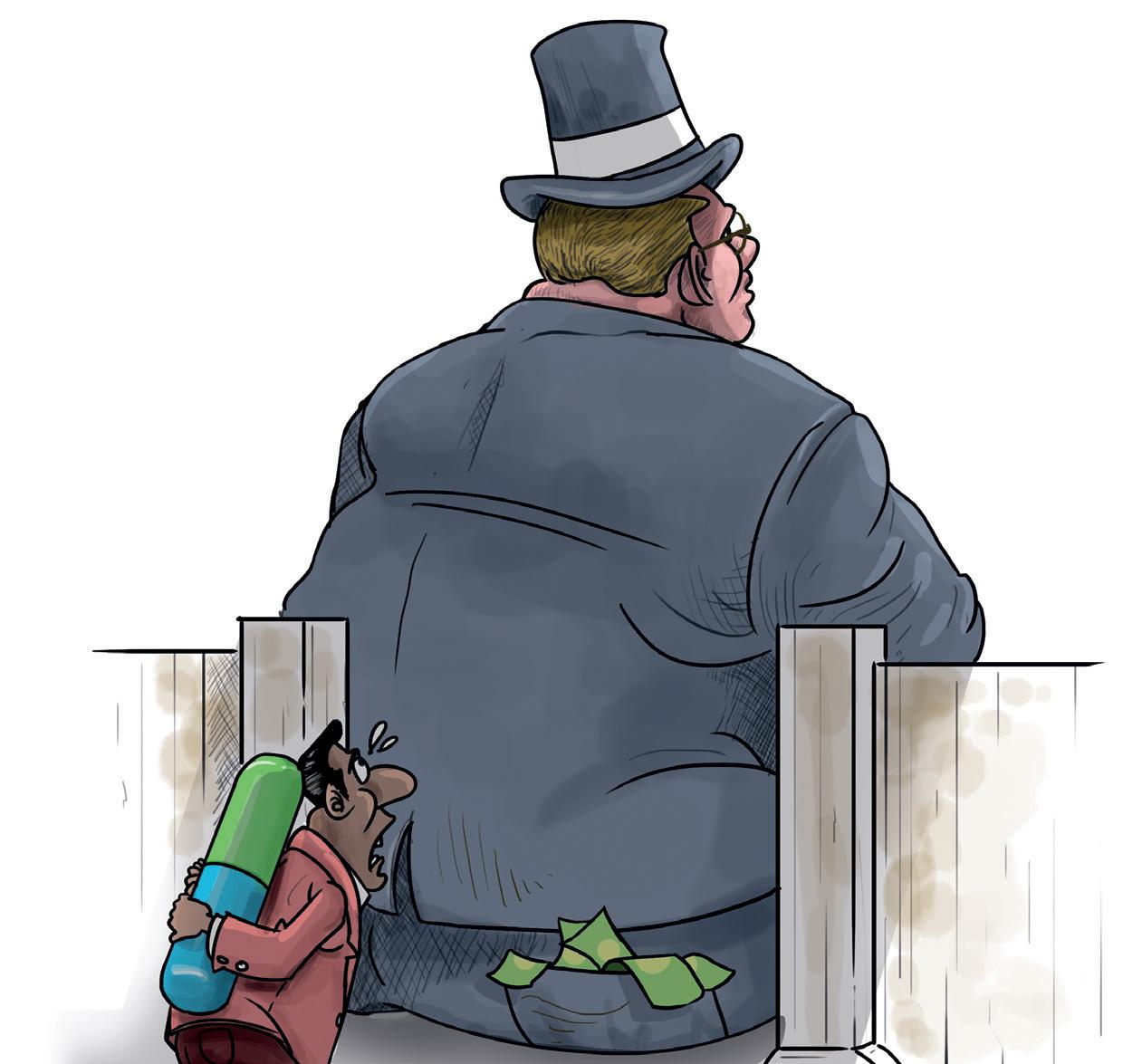
ONE'S HEART bleeds for American patients. They pay the most for prescription drugs compared to all other jurisdictions despite many ironies: a very large number of life-saving drugs are discovered by public-funded research in US universities and institutions; these pharmaceuticals are made in the US and by leading US-based manufacturers. Why is this so? It is because of a clutch of related reasons starting with the fact that there is no regulation of drug prices by the government but most of all, from exploiting a patent system that allows pharma companies to keep out competition and keep prices sky high. That's the root of the problem.
A case study of how drug companies manipulate the patent regime is AbbVie with its blockbuster anti-inflammation and arthritis drug Humira. The pharma giant has created a patent thicket around this top-selling biological medicine, by applying for an incredible 312 patents on this single drug and obtaining 166! And most of these patents (94 per cent) were sought after the drug was approved by the authorities. This has allowed AbbVie to garner revenues of US $208 billion since the patent was first granted in 2002. But here's the most disturbing aspect of Humira: two-thirds of this revenue was earned after its primary patents expired in 2016.
That was when patients and health insurance companies that pick up the tab for the drug were hoping to benefit from the less expensive biosimilar versions of the drug that were all set to launch in the US. But it did not happen. By shrewdly exploiting the US patent system, AbbVie blocked competitors from entering the market by using lawsuits against potential competitors and forcing them to accept settlements to delay their entry till 2023. The first of the biosimilar versions of Humira has only now been launched in the US, in February 2023-almost seven years after patent expiry.
This story is from the March 01, 2023 edition of Down To Earth.
Subscribe to Magzter GOLD to access thousands of curated premium stories, and 10,000+ magazines and newspapers.
Already a subscriber? Sign In
MORE STORIES FROM Down To Earth

Down To Earth
KING OF BIRDS
Revered for centuries, western tragopan now needs protection as its forests shrink, human pressures mount
3 mins
December 16, 2025

Down To Earth
WHISKERS ALL AQUIVER
Climate change threatens creatures that have weathered extreme environments for thousands of years
2 mins
December 16, 2025

Down To Earth
GOLDEN SPIRIT
Survival of the shy primate is closely tied to the health of Western Ghats
3 mins
December 16, 2025

Down To Earth
RINGED EYES IN THE CANOPY
Rapid habitat destruction forces arboreal langur to alter habits
2 mins
December 16, 2025

Down To Earth
HANGING BY THE CLIFF
The Himalaya's rarest wild goat is on the brink of local extinction
2 mins
December 16, 2025

Down To Earth
ANGEL OF THE BEAS
Conservation reserves, citizen science, and habitat protection give the Indus River dolphin a fighting chance in India
2 mins
December 16, 2025

Down To Earth
UNDER MOONLIT SCRUB
Survival of this hidden guardian tells us whether our scrublands still breathe
2 mins
December 16, 2025

Down To Earth
SYMBOL OF SILENT VALLEY
Lion-tailed macaque remains vulnerable despite past victories
2 mins
December 16, 2025

Down To Earth
THE APE IN OUR STORIES
India's only non-human ape species is a cultural icon threatened by forest fragmentation
2 mins
December 16, 2025

Down To Earth
SENTINEL OF THE HIGH COLD DESERT
The bird's evocative call may not continue to roll across the cold desert valley for long
3 mins
December 16, 2025
Translate
Change font size

