
The story of what might become the next major breakthrough in Covid-19 treatment starts on a hotel hallway floor in January 2020, months before you were worried about the virus, weeks before you likely knew it existed. A scientist and a business executive were at a health-care conference in San Francisco, hatching a plan to get a promising drug out of academia and into research trials for regulatory approval. George Painter, president of the Emory Institute for Drug Development, and Wendy Holman, chief executive officer of Ridgeback Biotherapeutics, had met at the Handlery Union Square Hotel to discuss a compound Painter had started developing with funding from the National Institutes of Health. They got so enthusiastic about the possibilities that their meeting ran long and a group of lawyers kicked them out of their room. So they continued on the hall floor, hours after they’d started.
Painter and Holman weren’t talking about targeting Covid at the time. The disease and the coronavirus that causes it, SARSCoV-2, weren’t major concerns at the J.P. Morgan-run conference, where handshakes and cocktail parties with hundreds of guests were still the norm. Rather, Painter was hoping his drug, molnupiravir, could get more funding to speed up flu studies. Holman was eager to see if it worked on Ebola. That’s the thing about molnupiravir: Many scientists think it could be a broad-spectrum antiviral, effective against a range of threats.
This story is from the March 29 - April 05, 2021 edition of Bloomberg Businessweek.
Start your 7-day Magzter GOLD free trial to access thousands of curated premium stories, and 8,500+ magazines and newspapers.
Already a subscriber ? Sign In
This story is from the March 29 - April 05, 2021 edition of Bloomberg Businessweek.
Start your 7-day Magzter GOLD free trial to access thousands of curated premium stories, and 8,500+ magazines and newspapers.
Already a subscriber? Sign In

Instagram's Founders Say It's Time for a New Social App
The rise of AI and the fall of Twitter could create opportunities for upstarts

Running in Circles
A subscription running shoe program aims to fight footwear waste

What I Learned Working at a Hawaiien Mega-Resort
Nine wild secrets from the staff at Turtle Bay, who have to manage everyone from haughty honeymooners to go-go-dancing golfers.

How Noma Will Blossom In Kyoto
The best restaurant in the world just began its second pop-up in Japan. Here's what's cooking

The Last-Mover Problem
A startup called Sennder is trying to bring an extremely tech-resistant industry into the age of apps

Tick Tock, TikTok
The US thinks the Chinese-owned social media app is a major national security risk. TikTok is running out of ways to avoid a ban
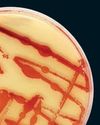
Cleaner Clothing Dye, Made From Bacteria
A UK company produces colors with less water than conventional methods and no toxic chemicals

Pumping Heat in Hamburg
The German port city plans to store hot water underground and bring it up to heat homes in the winter

Sustainability: Calamari's Climate Edge
Squid's ability to flourish in warmer waters makes it fitting for a diet for the changing environment

New Money, New Problems
In Naples, an influx of wealthy is displacing out-of-towners lower-income workers