試す 金 - 無料
"Biotech sector in Taiwan moves fast, making it easier to pivot and innovate"
BioSpectrum Asia
|March 2025
Caliway Biopharmaceuticals has recently announced the completion of its initial public offering (IPO) and up-listing from the Emerging Stock Market to the Taipei Exchange (TWSE-6919). The round raised approximately $206 million (NT$6.4 billion), marking it the largest IPO in Taiwan's biotech industry history and valuing the company at nearly $3 billion. The company is poised for a transformation in 2025, advancing its groundbreaking clinical programmes and strengthening its global market presence. Following its recent record-breaking IPO, BioSpectrum Asia took an opportunity to speak with Vivian Ling, Chief Executive Officer & Chief R&D Officer, Caliway Biopharmaceuticals to explore their innovative contributions in biopharmaceuticals. Edited excerpts:

Which products are currently under development?
2025 will be a defining year for Caliway as we push ahead with key clinical advancements and corporate milestones, bringing CBL-514 closer to market. CBL-514, a first-in-class small-molecule drug designed to selectively induce adipocyte apoptosis, provides a non-invasive alternative to liposuction for non-surgical fat reduction in medical aesthetics. We are preparing to secure IND approvals for two pivotal Phase 3 studies from the US FDA and Health Canada, a critical step in advancing CBL-514 as the world's first investigational drug for large-area localised fat reduction. Beyond fat reduction, we're also submitting a Phase 2 IND application for a new indication focused on improving body weight rebound, expanding CBL-514's potential applications.
In Q1, Caliway announced positive Phase 2b study results for CBL-514 (0205 Study), the second and final Phase 2b before moving into Phase 3. In Q2, we are preparing for regulatory discussions with the FDA (EOP2) and EMA to align on the next steps for CBL-514's late-stage development.
A key focus in Q4 will be completing patient enrollment for the Phase 2b study of CBL-514 in Dercum's Disease (CBL-514 0202DD). CBL-0202DD is being developed as a potential first-in-class therapy for Dercum's Disease, a rare and painful condition, and has already been granted Fast Track Designation by the FDA and Orphan Drug Designation by both the FDA and EMA. In the early-stage pipeline, CBA-539 offers a novel approach to hyperpigmentation and skin ageing by inhibiting melanin production and transmission, reducing dark spots and evening skin tone, while also stimulating collagen production to improve skin elasticity and firmness for natural, long-lasting results. Expanding into therapeutic applications, CBL-0201OB targets post-weight loss fat accumulation in combination with GLP-1, with a Phase 2 IND submission planned for Q4 2025.
このストーリーは、BioSpectrum Asia の March 2025 版からのものです。
Magzter GOLD を購読すると、厳選された何千ものプレミアム記事や、10,000 以上の雑誌や新聞にアクセスできます。
すでに購読者ですか? サインイン
BioSpectrum Asia からのその他のストーリー

BioSpectrum Asia
India signs MoU with Pfizer to strengthen healthcare innovation ecosystem
In a significant step towards strengthening India's healthcare innovation ecosystem, the Department for Promotion of Industry and Internal Trade (DPIIT), Ministry of Commerce and Industry, Government of India, has signed a Memorandum of Understanding (MoU) with US-based Pfizer Limited.
1 min
BioSpectrum Asia Oct 2025

BioSpectrum Asia
New Zealand to lead new gene therapy trial for muscular dystrophy
The first clinical trial of a new treatment for a rare form of muscular dystrophy is being led by New Zealand-based University of Auckland's Centre for Brain Research.
1 min
BioSpectrum Asia Oct 2025

BioSpectrum Asia
Scientists in Australia grow living skin in world-first
Australia's University of Queensland (UQ) researchers have been the first in the world to successfully grow fully functioning human skin in a laboratory.
1 min
BioSpectrum Asia Oct 2025

BioSpectrum Asia
India joins Health AI Global Regulatory Network to strengthen oversight of AI in healthcare
HealthAI - The Global Agency for Responsible AI in Health, has welcomed India as a pioneer country joining the HealthAI Global Regulatory Network (GRN), a global network of health regulators dedicated to the safe and effective use of artificial intelligence (AI) in healthcare.
1 min
BioSpectrum Asia Oct 2025

BioSpectrum Asia
TVM Capital Healthcare announces first closing of $150 M Southeast Asia Fund
Singapore-based TVM Capital Healthcare, a global private equity investor and operator specialised in healthcare growth capital across emerging markets, has announced the first closing of its $150 million TVM Healthcare Southeast Asia Fund (SEA Fund).
1 min
BioSpectrum Asia Oct 2025

BioSpectrum Asia
National Yang Ming Chiao Tung University, TSH Biopharm collaborate to foster pharma talent in Taiwan
Taiwan's National Yang Ming Chiao Tung University (NYCU) and TSH Biopharm have signed a five-year industry-academia collaboration Memorandum of Understanding (MOU).
1 min
BioSpectrum Asia Oct 2025

BioSpectrum Asia
Fujifilm Biosciences introduces BalanCD HEK293 Perfusion A Medium
Fujifilm Biosciences, a global leader in the innovation and manufacture of cell culture solutions for the life science market, has announced the commercial launch of BalanCD HEK293 Perfusion A.
1 min
BioSpectrum Asia Oct 2025
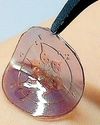
BioSpectrum Asia
Korea makes smart patch that can run tests using sweat instead of blood
A research team at Korea Advanced Institute of Science and Technology has developed a smart patch that can precisely observe internal changes through sweat when simply attached to the body.
1 min
BioSpectrum Asia Oct 2025

BioSpectrum Asia
ADB approves $106.9 M to strengthen secondary healthcare services in Sri Lanka
The Asian Development Bank (ADB) has approved a $106.9 million financing package to strengthen Sri Lanka's secondary curative care services, improve communicable disease prevention and control, and enhance the healthcare sector's governance and management capacity.
1 min
BioSpectrum Asia Oct 2025

BioSpectrum Asia
Epigenic Therapeutics raises $60 M to accelerate clinical development of medicines
China-based startup Epigenic Therapeutics, a clinical-stage innovative drug development company, has announced the completion of a $60 million Series B round of financing led by Lapam Capital, with continued participation from existing investors Qiming Venture Partners and OrbiMed, and addition of new investors including IFSC and a renowned investment firm in the industry.
1 min
BioSpectrum Asia Oct 2025
Listen
Translate
Change font size

