
On a late August day in an industrial corner of Baltimore that had been mostly silenced by the pandemic, a redbrick manufacturing plant was buzzing with activity. Deep in the building, in a zone called Area 3, the stainless steel shell of a bioreactor lay on its side, having just arrived from Massachusetts. Employees had begun the task of making the bioreactor operational. Within weeks it would be the center of a production line for coronavirus vaccines.
When the owner of the plant, Emergent BioSolutions Inc., ordered the bioreactor, one supplier said some critical parts wouldn’t arrive until November or December. And so Emergent enlisted the help of Operation Warp Speed, the federal government’s mission to accelerate development of a Covid-19 vaccine. Officials working with OWS, a couple of whom are embedded with Emergent, called the supplier to say the order couldn’t wait, throwing the weight of the government behind the request. “It’s almost like having that Bat-Signal,” says Syed Husain, who heads the company’s contract manufacturing business. “They’ve been a great partner for us.”
This story is from the November 02, 2020 edition of Bloomberg Businessweek.
Start your 7-day Magzter GOLD free trial to access thousands of curated premium stories, and 8,500+ magazines and newspapers.
Already a subscriber ? Sign In
This story is from the November 02, 2020 edition of Bloomberg Businessweek.
Start your 7-day Magzter GOLD free trial to access thousands of curated premium stories, and 8,500+ magazines and newspapers.
Already a subscriber? Sign In

Instagram's Founders Say It's Time for a New Social App
The rise of AI and the fall of Twitter could create opportunities for upstarts

Running in Circles
A subscription running shoe program aims to fight footwear waste

What I Learned Working at a Hawaiien Mega-Resort
Nine wild secrets from the staff at Turtle Bay, who have to manage everyone from haughty honeymooners to go-go-dancing golfers.

How Noma Will Blossom In Kyoto
The best restaurant in the world just began its second pop-up in Japan. Here's what's cooking

The Last-Mover Problem
A startup called Sennder is trying to bring an extremely tech-resistant industry into the age of apps

Tick Tock, TikTok
The US thinks the Chinese-owned social media app is a major national security risk. TikTok is running out of ways to avoid a ban
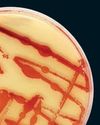
Cleaner Clothing Dye, Made From Bacteria
A UK company produces colors with less water than conventional methods and no toxic chemicals

Pumping Heat in Hamburg
The German port city plans to store hot water underground and bring it up to heat homes in the winter

Sustainability: Calamari's Climate Edge
Squid's ability to flourish in warmer waters makes it fitting for a diet for the changing environment

New Money, New Problems
In Naples, an influx of wealthy is displacing out-of-towners lower-income workers